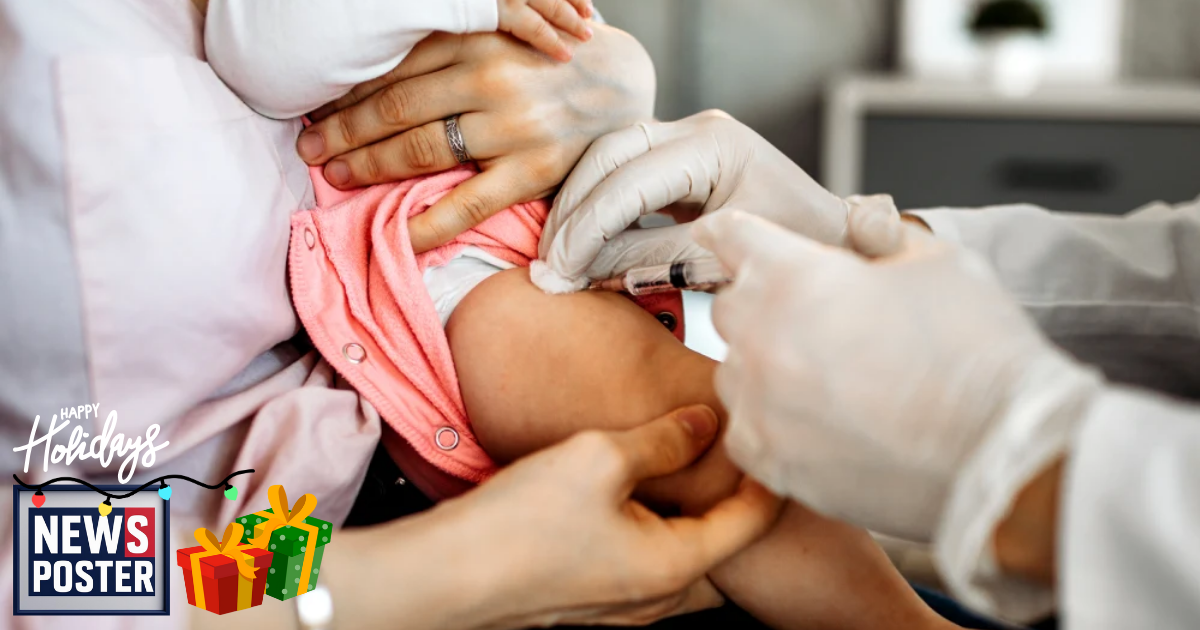
Advisers to the federal public health agency are preparing to vote on whether to overturn a decades-old recommendation that all newborns receive a hepatitis B vaccination within 24 hours of birth. The policy, in place since the early 1990s, is widely credited with slashing acute hepatitis B infections in children by 99%. Hepatitis B is a serious viral infection that can be transmitted from mother to child during childbirth and can result in chronic liver disease, liver cancer, and early death. There is no cure. Despite the vaccine’s long track record of success, it has become the latest focus of growing skepticism from some physicians and policymakers who argue that potential risks may outweigh benefits for low-risk infants. One committee member has raised concerns that giving the vaccine on the first day of life may lead to fevers that trigger additional medical testing. However, pediatric infectious disease specialists counter that serious vaccine-related reactions in newborns are extremely rare. Large-scale reviews of hundreds of studies have found no evidence that the birth dose causes short- or long-term harm and estimate that newborn vaccination has prevented millions of infections and nearly a million hospitalizations.
The advisory committee is scheduled to meet later this week, where members will debate whether to eliminate or delay the first dose by several weeks or months. While any recommendation would not ban doctors from giving the shot, it would strongly influence insurance coverage and hospital practices nationwide. Supporters of maintaining the universal birth dose warn that relying on maternal screening alone is risky, as not all pregnant women receive prenatal care or are fully screened for hepatitis B. Without vaccination, as many as 90% of infants exposed during delivery develop chronic infection. The debate is unfolding amid broader internal turmoil at the agency, following leadership changes, restructuring, and revisions to long-standing public vaccine messaging. The meeting is also expected to include a discussion of aluminum-based vaccine ingredients, which large population studies have found are not linked to increased risks of chronic illness. The outcome of the vote could reshape U.S. newborn vaccination policy for the first time in more than 30 years and carries major implications for public health protections for infants.