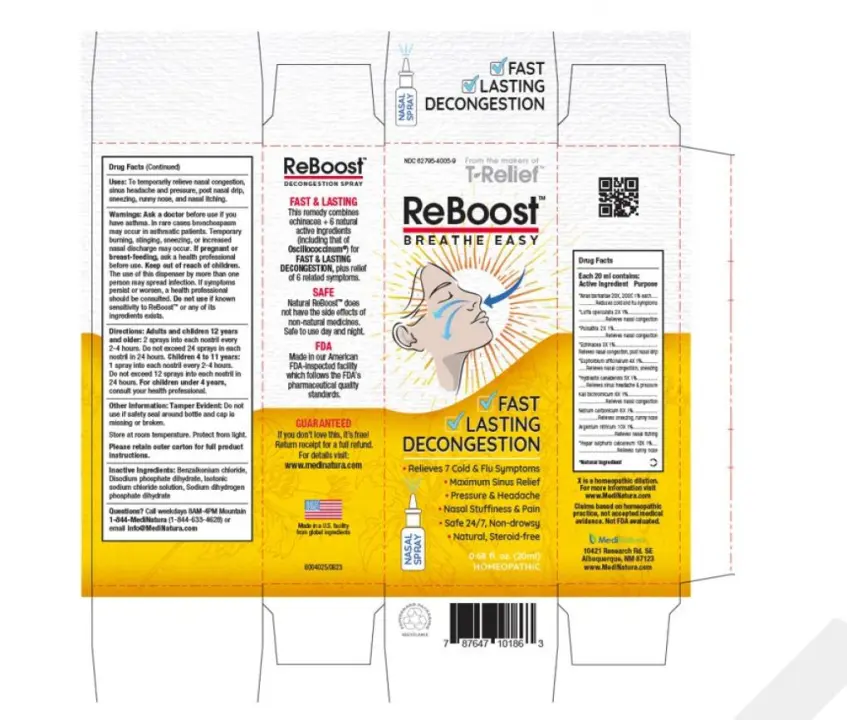
The FDA has issued a warning about a recall of ReBoost Nasal Spray due to microbial contamination. MediNatura New Mexico voluntarily recalled a single lot of the homeopathic spray after tests revealed the presence of yeast, mold, and the bacterium Achromobacter. These contaminants could pose serious health risks, particularly for people with weakened immune systems.
MediNatura confirmed that no adverse events have been reported in connection with this recall. ReBoost Nasal Spray is marketed to temporarily relieve nasal congestion, sinus headaches, pressure, postnasal drip, sneezing, runny nose, and nasal itching. It comes in a 20 mL bottle packaged in a white-and-yellow carton. The recalled lot carries the NDC number 62795-4005-9, UPC 787647101863, lot number 224268, and expires in December 2027. The product was distributed nationwide through retail stores and online. Consumers are advised to stop using the recalled nasal spray immediately. Those who purchased directly from MediNatura can request a refund via recall@medinatura.com, while products purchased from retailers should be returned to the store.